| |
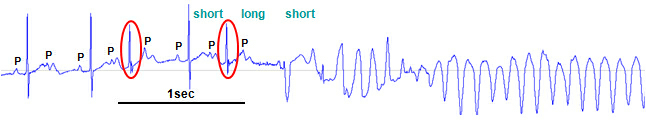
We have generated transgenic long-QT syndrome type 1 and type 2 rabbits over-expressing dominant-negative loss-of-function pore mutants of the human repolarizing K+ channels KvLQT1 (LQT1) or HERG (LQT2) in a cooperative project with Brown University (Cardiovascular Research Center, Providence, RI, USA) and Penn State University (Comparative Medicine, Hershey, PA, USA).
These LQT1 and LQT2 rabbits are the first genetic models for long-QT syndrome to mimic the human LQTS phenotype with QT interval prolongation, steeper QT/RR ratio in females, spontaneous polymorphic ventricular tachycardia, and sudden cardiac death (SCD) - with a particularly high incidence of SCD in the postpartum period. We are using this transgenic model to investigate how extrinsic and intrinsic factors - such as hormones or drugs - modulate the arrhythmogenic phenotype and how these findings can help us in treating LQTS patients.

 Pro- and anti-arrhythmic hormone effects in LQT2 Pro- and anti-arrhythmic hormone effects in LQT2
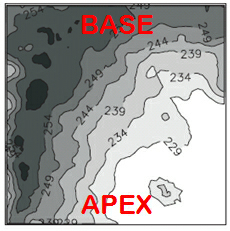 Women with inherited long-QT syndrome 2 (LQT2) are at higher risk for polymorphic ventricular tachycardia and sudden cardiac death than men and exhibit a particularly high arrhythmia incidence during the postpartum period. We have elucidated that sex hormones modulate the arrhythmogenic risk in transgenic LQT2 rabbits: estradiol exerts a pro-arrhythmic effect and progesterone and testosterone exert an anti-arrhythmic, protective effect in LQT2 syndrome. Currently, we are investigating the arrhythmogenic effects of the postpartum hormones oxytocin and prolactin and their role in postpartum arrhythmogenesis. Women with inherited long-QT syndrome 2 (LQT2) are at higher risk for polymorphic ventricular tachycardia and sudden cardiac death than men and exhibit a particularly high arrhythmia incidence during the postpartum period. We have elucidated that sex hormones modulate the arrhythmogenic risk in transgenic LQT2 rabbits: estradiol exerts a pro-arrhythmic effect and progesterone and testosterone exert an anti-arrhythmic, protective effect in LQT2 syndrome. Currently, we are investigating the arrhythmogenic effects of the postpartum hormones oxytocin and prolactin and their role in postpartum arrhythmogenesis.
This work is in collaboration with the Prof. Gideon Koren and Prof. Bum-Rak Choi both at Brown University, Providence, USA,
and Prof. Xuwen Peng at
Penn State University, Hershey, USA. It is funded by the German Research Foundation (DFG), the German Cardiac Society (DGK), the American Heart Association (AHA), and the Prof. Just Foundation (Heilmeyer Stipendium).

Mechanisms of arrhythmogenesis in transgenic short-QT syndrome rabbits
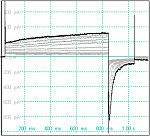 We are currently in the process of generating transgenic short-QT syndrome (SQTS) rabbit models with gain-of-function mutations in repolarizing cardiac K+ channels. SQTS is an inherited arrhythmogenic disease in which a pathological shortening of cardiac repolarization confers an arrhythmogenic phenotype. Patients are not only prone to polymorphic VTs and sudden cardiac death but also develop atrial fibrillation - the most common arrhythmia in humans. We will use this transgenic model to investigate the molecular mechanisms of arrhythmogenesis in SQTS and to identify pro-arrhythmic triggers. We are currently in the process of generating transgenic short-QT syndrome (SQTS) rabbit models with gain-of-function mutations in repolarizing cardiac K+ channels. SQTS is an inherited arrhythmogenic disease in which a pathological shortening of cardiac repolarization confers an arrhythmogenic phenotype. Patients are not only prone to polymorphic VTs and sudden cardiac death but also develop atrial fibrillation - the most common arrhythmia in humans. We will use this transgenic model to investigate the molecular mechanisms of arrhythmogenesis in SQTS and to identify pro-arrhythmic triggers.
This work is in collaboration with Dr. Genevieve Jolivet at the INRA, Jouy-en-Josas, France, and Prof. Axel zur Hausen
Dept. of Pathology, Maastricht, NL. It is funded by the German Research Foundation (DFG).

Electromechanical dysfunction in LQTS - image-based bio-physical modeling
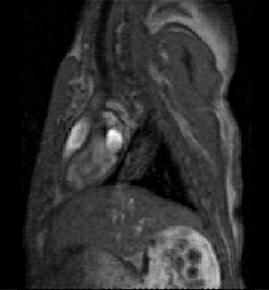 We aim at elucidating how the impaired cardiac repolarization in LQTS affects mechanical function using magnetic resonance imaging methods to determine regional differences in contraction and relaxation velocities. Using these data on electrical and mechanical function, we aim at generating an electromechanical model of the LQTS heart. We aim at elucidating how the impaired cardiac repolarization in LQTS affects mechanical function using magnetic resonance imaging methods to determine regional differences in contraction and relaxation velocities. Using these data on electrical and mechanical function, we aim at generating an electromechanical model of the LQTS heart.
This work is in collaboration with Dr. Daniela Foell, Cardiac MRI, Dept. of Cardiology, Freiburg, and Dr. Bernd Jung, Medical Physics, Freiburg, and Prof. Dr. Maxime Sermesant at the INRIA, Sophia-Antipolis, France, and King's College, London, UK.

Drug-effects on cardiac repolarization in LQTS
 The acquired drug-induced LQTS is a major and potentially fatal adverse drug-effect of a variety of different drug classes (such as anti-depressant, anti-psychotic, or antibiotic drugs) that has already caused the withdrawal of several widely used drugs from the market. However, not only the "classical" IKr blocking agents but also IKs blocking drugs can exert pro-arrhythmic effects in subjects with a reduced repolarization reserve. We aim at establishing whether the transgenic LQT1 and LQT2 rabbits could serve as an in vivo model to assess QT prolonging and QT shortening properties of drugs and to differentiate the arrhythmogenic risk of different ion channel blocking or activating agents. The acquired drug-induced LQTS is a major and potentially fatal adverse drug-effect of a variety of different drug classes (such as anti-depressant, anti-psychotic, or antibiotic drugs) that has already caused the withdrawal of several widely used drugs from the market. However, not only the "classical" IKr blocking agents but also IKs blocking drugs can exert pro-arrhythmic effects in subjects with a reduced repolarization reserve. We aim at establishing whether the transgenic LQT1 and LQT2 rabbits could serve as an in vivo model to assess QT prolonging and QT shortening properties of drugs and to differentiate the arrhythmogenic risk of different ion channel blocking or activating agents.
This work is in collaboration with
Prof. Morten Grunnet, Danish Arrhythmia Research Center, University of Copenhagen, Denmark.
[ back to top ]
|
|




 Women with inherited long-QT syndrome 2 (LQT2) are at higher risk for polymorphic ventricular tachycardia and sudden cardiac death than men and exhibit a particularly high arrhythmia incidence during the postpartum period. We have elucidated that sex hormones modulate the arrhythmogenic risk in transgenic LQT2 rabbits: estradiol exerts a pro-arrhythmic effect and progesterone and testosterone exert an anti-arrhythmic, protective effect in LQT2 syndrome. Currently, we are investigating the arrhythmogenic effects of the postpartum hormones oxytocin and prolactin and their role in postpartum arrhythmogenesis.
Women with inherited long-QT syndrome 2 (LQT2) are at higher risk for polymorphic ventricular tachycardia and sudden cardiac death than men and exhibit a particularly high arrhythmia incidence during the postpartum period. We have elucidated that sex hormones modulate the arrhythmogenic risk in transgenic LQT2 rabbits: estradiol exerts a pro-arrhythmic effect and progesterone and testosterone exert an anti-arrhythmic, protective effect in LQT2 syndrome. Currently, we are investigating the arrhythmogenic effects of the postpartum hormones oxytocin and prolactin and their role in postpartum arrhythmogenesis.  We are currently in the process of generating transgenic short-QT syndrome (SQTS) rabbit models with gain-of-function mutations in repolarizing cardiac K+ channels. SQTS is an inherited arrhythmogenic disease in which a pathological shortening of cardiac repolarization confers an arrhythmogenic phenotype. Patients are not only prone to polymorphic VTs and sudden cardiac death but also develop atrial fibrillation - the most common arrhythmia in humans. We will use this transgenic model to investigate the molecular mechanisms of arrhythmogenesis in SQTS and to identify pro-arrhythmic triggers.
We are currently in the process of generating transgenic short-QT syndrome (SQTS) rabbit models with gain-of-function mutations in repolarizing cardiac K+ channels. SQTS is an inherited arrhythmogenic disease in which a pathological shortening of cardiac repolarization confers an arrhythmogenic phenotype. Patients are not only prone to polymorphic VTs and sudden cardiac death but also develop atrial fibrillation - the most common arrhythmia in humans. We will use this transgenic model to investigate the molecular mechanisms of arrhythmogenesis in SQTS and to identify pro-arrhythmic triggers.  We aim at elucidating how the impaired cardiac repolarization in LQTS affects mechanical function using magnetic resonance imaging methods to determine regional differences in contraction and relaxation velocities. Using these data on electrical and mechanical function, we aim at generating an electromechanical model of the LQTS heart.
We aim at elucidating how the impaired cardiac repolarization in LQTS affects mechanical function using magnetic resonance imaging methods to determine regional differences in contraction and relaxation velocities. Using these data on electrical and mechanical function, we aim at generating an electromechanical model of the LQTS heart.  The acquired drug-induced LQTS is a major and potentially fatal adverse drug-effect of a variety of different drug classes (such as anti-depressant, anti-psychotic, or antibiotic drugs) that has already caused the withdrawal of several widely used drugs from the market. However, not only the "classical" IKr blocking agents but also IKs blocking drugs can exert pro-arrhythmic effects in subjects with a reduced repolarization reserve. We aim at establishing whether the transgenic LQT1 and LQT2 rabbits could serve as an in vivo model to assess QT prolonging and QT shortening properties of drugs and to differentiate the arrhythmogenic risk of different ion channel blocking or activating agents.
The acquired drug-induced LQTS is a major and potentially fatal adverse drug-effect of a variety of different drug classes (such as anti-depressant, anti-psychotic, or antibiotic drugs) that has already caused the withdrawal of several widely used drugs from the market. However, not only the "classical" IKr blocking agents but also IKs blocking drugs can exert pro-arrhythmic effects in subjects with a reduced repolarization reserve. We aim at establishing whether the transgenic LQT1 and LQT2 rabbits could serve as an in vivo model to assess QT prolonging and QT shortening properties of drugs and to differentiate the arrhythmogenic risk of different ion channel blocking or activating agents.